Clinical data of ADPS™
31% higher detection rate than company “R”.
The performance of ADPS™ has been confirmed by several clinical trials.
The data below is a clinical performance evaluation of the ADPS™ EGFR Mutation Test kit compared to previously approved products at Seoul Asan Medical Center in November 2019.
40 participants who had been diagnosed with NSCLC (adenocarcinoma or squamous cell carcinoma) participated. Before the clinical trial, all 40 participants were confirmed as positive or negative for EGFR T790M and C797S by tissue biopsy. (The samples in this test were voluntarily provided by the patients with written consent.)
Data of sample analysis by liquid biopsy ADPS™ versus “R” company
| Sample# | ADPS™ results | Determinaion | ||||
|---|---|---|---|---|---|---|
| Input copies/rxn | Detected copies | MAF(%) | ADPS™ | 'R' Company | Amplicon based NGS evalutation | |
| #15 | 949 | 17 | 1.82 | + | - | + |
| #20 | 1,102 | 2 | 0.15 | + | - | + |
| #27 | 41 | 1 | 3.42 | + | - | + |
| #33 | 2,163 | 9 | 0.43 | + | - | + |
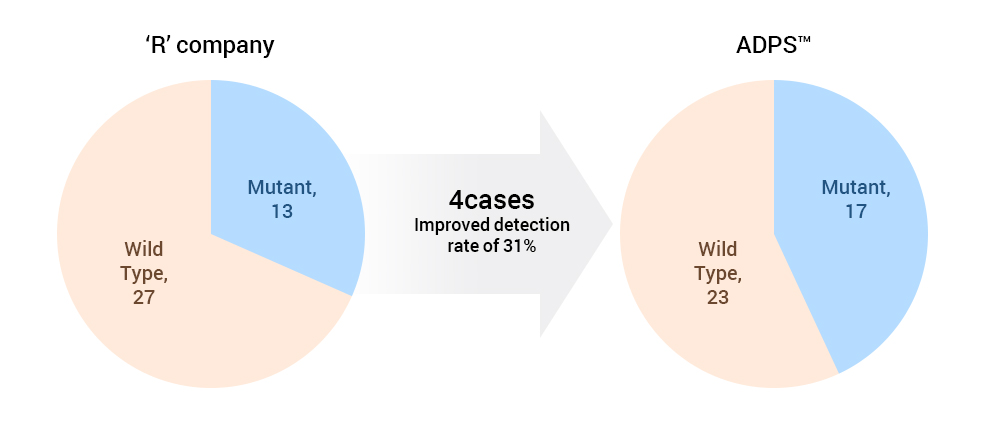
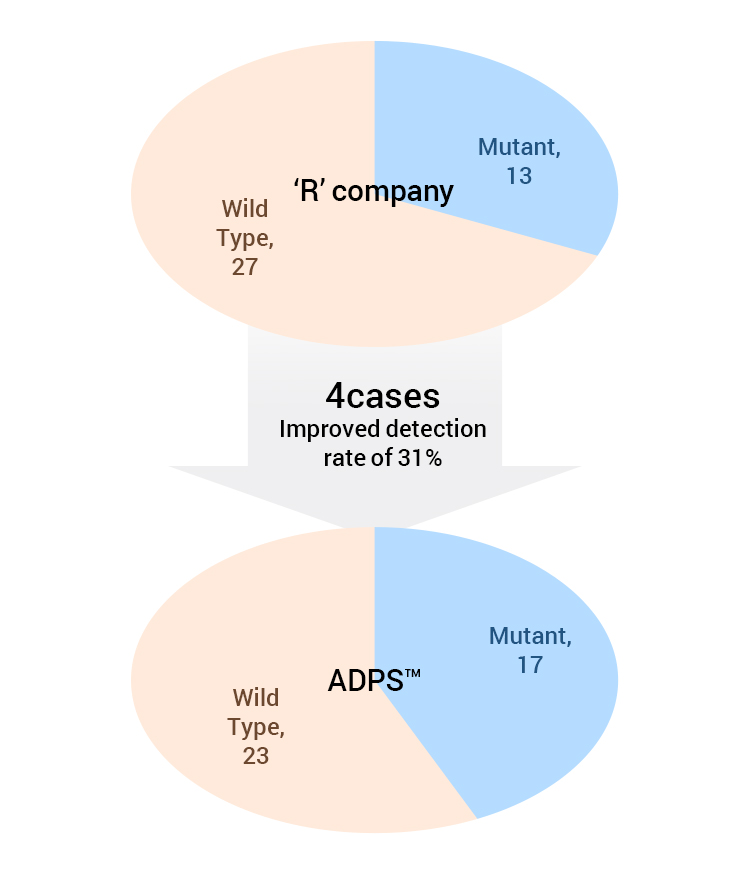
| T790M(Plasma) | 'R' Company | Total | ||
|---|---|---|---|---|
| Mutation | Normal | |||
| GENECAST ADPS™ | Mutation | 13 | 4 | 17 |
| Normal | 0 | 23 | 23 | |
| Total | 13 | 27 | 40 | |
| PPA (Positive percent agreement) | 100.0% | 13/13x100 | ||
| NPA (Negative percent agreement) | 85.2% | 23/27x100 | ||
| OPA (Overall percent agreement) | 90.0% | (13+23)/40x100 | ||
| T790M(Plasma) | 'R' Company + NGS | Total | ||
|---|---|---|---|---|
| Mutation | Normal | |||
| GENECAST ADPS™ | Mutation | 17 | 0 | 17 |
| Normal | 0 | 23 | 23 | |
| Total | 17 | 23 | 40 | |
| PPA (Positive percent agreement) | 100.0% | 17/17x100 | ||
| NPA (Negative percent agreement) | 100.0% | 23/23x100 | ||
| OPA (Overall percent agreement) | 100.0% | (17+23)/40x100 | ||
GENECAST conducted a comparison experiment between ADPS™ EGFR Mutation Test Kit v1 and EGFR Mutation Test kit of company “R” at Asan Medical Center, one of the biggest hospitals in Korea. After the experiment, NGS was selected for verification.
As a result, ADPS™ EGFR Mutation Test Kit v1 results coincided with NGS while the EGFR Mutation Test kit of company “R” showed 4 false-negatives.